How I improved my class performance on the ACS organic chemistry examination
Exams without mechanisms had higher scores
I noted that scores on the first organic chemistry exam were higher than all others. The last regular semester exam usually had the lowest scores. I believe this result is consistent with the role that understanding and predicting reactions has on learning organic chemistry. Most organic chemistry textbooks open with principles of electronegativity, hybridization, nomenclature, isomers, conformations, etc. These items are static in nature. Students are simply required to learn the appropriate terms. Reactions are inherently dynamic in nature. It is insufficient to recognize static qualities of the reactants, but must apply those qualities into a dynamic process in which the reactant becomes transformed into a product. Qualitatively, that presents a greater challenge and could explain low scores late in the course. If an exam contained more points from reaction mechanisms, it resulted in lower scores.
I thought of my experience in learning organic chemistry. My professors illustrated reaction mechanisms and I was required to repeat that mechanism on an exam. I knew that I needed to be able to write at least one example of a reaction mechanism if I was to use it to solve a new problem. I wrote reaction mechanisms in explaining how a product should form. I also wrote a number of reaction mechanisms for problems in solutions manuals. However, I found little evidence of students applying mechanistic thinking to problems on their quizzes or exams.
Gave five mechanisms to class before exam
I was determined to require the class to learn reaction mechanisms. Two weeks before an exam, I handed out reaction mechanisms to five problems that were on the next exam. I explained that since the class had the problems in advance, that partial credit was going to be limited. The five problems totaled thirty points. The minimum score for each problem was at least three points. I anticipated this to be a good trade with the class. They could earn exam points upon learning the mechanisms, and it would build mechanistic understanding.
I was unprepared to discover that nearly one half of the class had zero points on these problems. It would have been easy to blame the class for their lack of accomplishment, but it proved to be an opportunity to reflect upon how students think. Why should the mechanisms be difficult to understand? How could I improve student’s ability to remember the mechanisms?
Why are mechanisms hard to remember?
After thinking about the possible reasons, the answer that I think best describes the failure is as follows. If someone asked you to write the Gettysburg Address, it would be tedious, but you could do it. If they asked you to write it in German, it would be more difficult, but since the words are derived from the same root, you could still write it. However, if you were asked to write it in Chinese (presumably a language you did not know), it would change from tedious to impossible. The difference is that the logic that linked the words and characters would be lost.
In the process of memorizing something, the use of a mnemonic is common. For chemists, they may use GET RAXL or Oh My Such Good Apple Pie to remember the names of the first seven sugars or the first six dicarboxylic acids. These are effective because it creates a logical connection between the first letter of the mnemonic to the chemical. Without the mnemonic, there is no connection to the names and they are more difficult to recall. I believe the failure of students to memorize the mechanisms may result from a failure to connect the mechanisms with a logic.
In order to improve learning organic chemistry, I sought a better strategy. I began by thinking about how the brain works and what does it do? I believe that our brains are fundamentally pattern matching machines. For example, the French language class I took in college used matching dialogs in French and English. The dialog could be listened to in the language lab and the English equivalent could be noted while listening. This enabled connecting the spoken French words to an English meaning. Similar connections can be thought of in teaching a dog to sit for example. That is, connecting the command to sit with the action of sitting (and receiving a reward for doing so).
How to make mechanisms understandable
Assuming that our brains are pattern matching machines, then clarity, repetition and consistency should enable students to learn the patterns. Conversely, ambiguity and exceptions might confuse students.
I thought about how mechanisms are written and what students might understand. Consider the Markovnikov addition of hydrogen chloride to propene. This is a commonly illustrated in organic chemistry textbooks. The proton adds to the least substituted carbon and the intermediate is the most stable carbocation. However, what part of the curved arrow tells you this? I suggest that for a student to understand what the product will be from the curved arrows, they must already know what the intermediate/product is. The curved arrows are not aiding a student to predict a product.
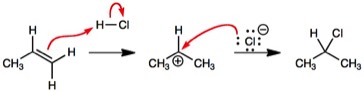
I sought a method to make a product to be logically predicted from the curved arrows. An obvious solution would be to use a dashed line to indicate where a bond will form. I first noted this usage in “Macroscale and Microscale Organic Experiments” by Williamson. In 1957, Zimmerman and Traxler used dashed lines with arrow heads to indicate where bonds were to form in aldol condensation reactions.
Adding a dashed line to indicate where a bond will form (a ‘pre-bond’) renders the curved arrows unambiguous and makes their usage consistent with, e.g., resonance structures. In resonance structures, curved arrows always end either on an atom if a bond breaks or on a bond to change the bond order. With a pre-bond, one should be able to draw the intermediate/product of any reaction from the curved arrows. I used this in my class to explain reaction mechanisms with wording such as, “I am going to form a bond between the carbon and hydrogen” and drew a dashed line/pre-bond. I then added the phrase, “with this pair of electrons” and drew the curved arrow. I then added I would “break the HCl bond” and drew the other curved arrow.
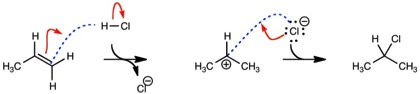
To Make a Bond or Increase the Bond Order
To Break a Bond or Reduce the Bond Order
By adding ‘pre-bonds’ to mechanisms, they are now unambiguous. Therefore, this example can be applied as a teaching model. If the curved arrows are not present, a student can still logically add them. If the same problem was repeated, but with some information missing, a student could repeat the reaction using the same logic. Finally, the same problem can be repeated without any of the aids.
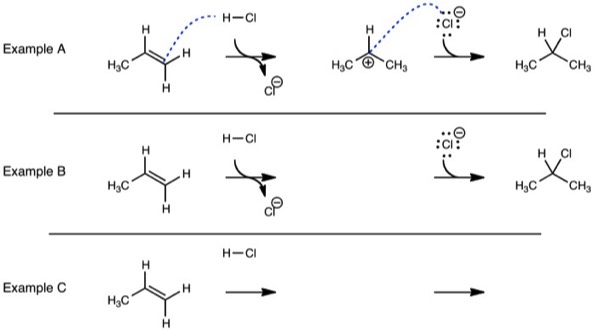
I began writing worksheets similar to Examples A, B, and C. Because the examples could be solved by students without further guidance, I used them in class virtually in lieu of lectures. While these problems are seemingly easy, they contained the elements necessary for understanding reaction mechanisms. They model the electron flow that leads from starting materials to products. Rather than students copying information for later reference, they are rehearsing the steps they must use in solving similar problems.
Because I had small classes, I had students work in assigned groups. No additional rules were applied to the groups. I encouraged students to help each other. I moved about the class observing student progress and making additional comments as needed. In order to maintain the pace of the class, I mainly did problems similar to Examples A and B, and only rarely of Example C.
I believe I succeeded in enabling students to write reaction mechanisms. I continued to assign five to seven reaction mechanisms before each exam. I arranged class topics so that reaction mechanisms were included on all exams. All quizzes and exams had problems requiring students to write a mechanism for a problem. A problem may or may not have a product present, depending on whether I thought the product may have been obvious or not. As a result, I noted more students using mechanistic thinking whether it was a required for an answer or not.
