Curved arrows have evolved to explain chemical reactions. I wanted curved arrows that showed which electrons are moving and what bonds are made or broken. A curved arrow must start with a pair of electrons and end either between two atoms (to make a bond) or on an atom (to break a bond). In order to make predicting the product of a reaction unambiguous, I adopted a 'pre-bond' (a dashed line) to represent where a new bond will form, see for example, Williamson, Chapter 49, Diels-Alder Reaction in Macroscale and Microscale Organic Reactions (Houghton Miflin, 4th edition).
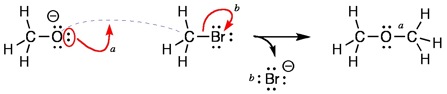
In the example below, we may write the following sentences to represent the electron movements represented by the curved arrows 'a' and 'b'. For curved arrow 'a', "A new bond will be formed between the oxygen atom and carbon atom. This is the 'pre-bond' (dashed line)." The non-bonded electrons on the oxygen atoms are used to make the new bond. For curved arrow 'b', "Concurrently, the bond between the carbon and bromine atom is broken with the electrons remaining attached to the bromine." The new bond and the non-bonded electrons that were represented by the curved arrows are noted in the products.
The issue for use of 'pre-bonds' should not be a question of whether it conforms to prior usage, but how clearly the meaning of the curved arrows is carried. In class, I found it very natural to describe bond formation with the English sentences as I indicated. Writing reaction mechanisms with pre-bonds results in an easy to understand mechanism.
In the example below, we may write the following sentences to represent the electron movements represented by the curved arrows 'a' and 'b'. For curved arrow 'a', "A new bond will be formed between the oxygen atom and carbon atom. This is the 'pre-bond' (dashed line)." The non-bonded electrons on the oxygen atoms are used to make the new bond. For curved arrow 'b', "Concurrently, the bond between the carbon and bromine atom is broken with the electrons remaining attached to the bromine." The new bond and the non-bonded electrons that were represented by the curved arrows are noted in the products.
The issue for use of 'pre-bonds' should not be a question of whether it conforms to prior usage, but how clearly the meaning of the curved arrows is carried. In class, I found it very natural to describe bond formation with the English sentences as I indicated. Writing reaction mechanisms with pre-bonds results in an easy to understand mechanism.